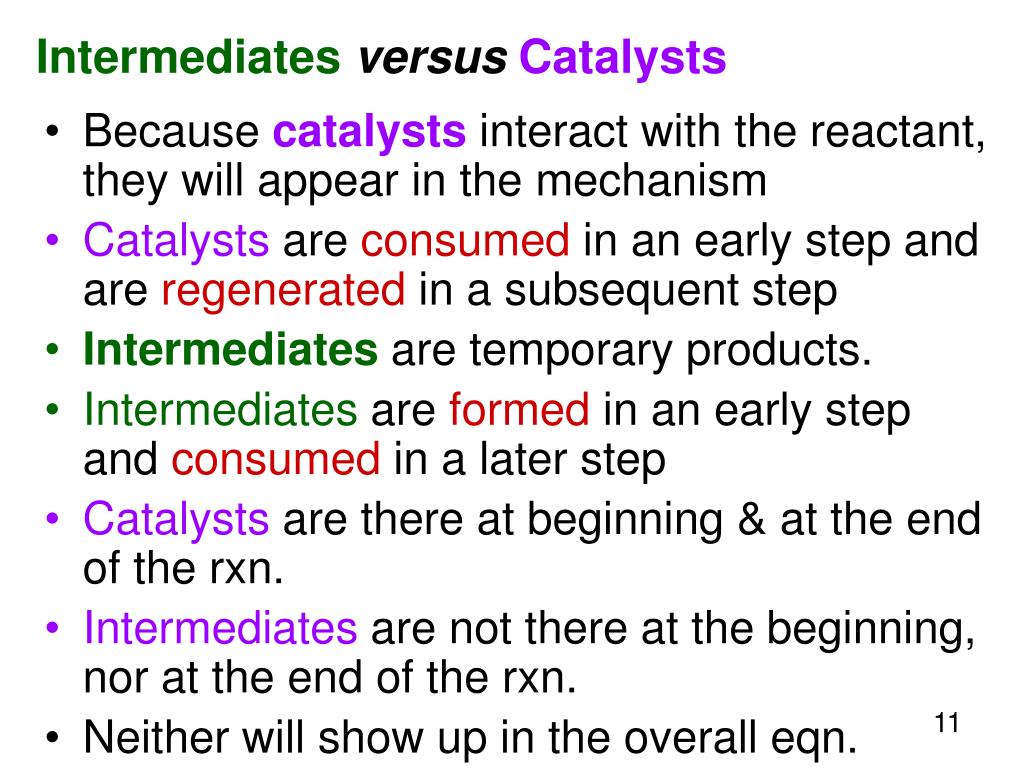
Difference Between Intermediates And Catalysts . Catalysts often react with reactants to form intermediates that eventually yield the same reaction products and regenerate the catalyst. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are. Draw a reaction energy diagram for a given multistep process. describe the similarities and differences between the three principal classes of catalysts. goes over two examples that highlight the differences between catalysts and intermediates. while catalysts and intermediates both participate in chemical reactions, they have distinct roles and characteristics. A catalyst is used at. the main difference between a catalyst and an intermediate is their role and status in a chemical reaction. a catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. explain the difference between a transition state and an intermediate.
from www.slideserve.com
the main difference between a catalyst and an intermediate is their role and status in a chemical reaction. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are. explain the difference between a transition state and an intermediate. Catalysts often react with reactants to form intermediates that eventually yield the same reaction products and regenerate the catalyst. Draw a reaction energy diagram for a given multistep process. A catalyst is used at. goes over two examples that highlight the differences between catalysts and intermediates. while catalysts and intermediates both participate in chemical reactions, they have distinct roles and characteristics. describe the similarities and differences between the three principal classes of catalysts. a catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity.
PPT Part V Reaction Mechanisms PowerPoint Presentation
Difference Between Intermediates And Catalysts goes over two examples that highlight the differences between catalysts and intermediates. a catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. describe the similarities and differences between the three principal classes of catalysts. Catalysts often react with reactants to form intermediates that eventually yield the same reaction products and regenerate the catalyst. the main difference between a catalyst and an intermediate is their role and status in a chemical reaction. Draw a reaction energy diagram for a given multistep process. explain the difference between a transition state and an intermediate. A catalyst is used at. goes over two examples that highlight the differences between catalysts and intermediates. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are. while catalysts and intermediates both participate in chemical reactions, they have distinct roles and characteristics.
From courses.lumenlearning.com
Catalysis Chemistry Difference Between Intermediates And Catalysts Catalysts often react with reactants to form intermediates that eventually yield the same reaction products and regenerate the catalyst. Draw a reaction energy diagram for a given multistep process. describe the similarities and differences between the three principal classes of catalysts. a catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate. Difference Between Intermediates And Catalysts.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free Difference Between Intermediates And Catalysts in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are. Catalysts often react with reactants to form intermediates that eventually yield the same reaction products and regenerate the catalyst. while catalysts and intermediates both participate in chemical reactions, they have distinct roles and characteristics. Draw a reaction energy diagram. Difference Between Intermediates And Catalysts.
From www.chegg.com
Solved Identify The Reactant/s, Product/s, Intermediate/s... Difference Between Intermediates And Catalysts in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are. describe the similarities and differences between the three principal classes of catalysts. goes over two examples that highlight the differences between catalysts and intermediates. Catalysts often react with reactants to form intermediates that eventually yield the same reaction. Difference Between Intermediates And Catalysts.
From chemistnotes.com
Reaction Intermediates, Example, and Types Chemistry Notes Difference Between Intermediates And Catalysts in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are. the main difference between a catalyst and an intermediate is their role and status in a chemical reaction. while catalysts and intermediates both participate in chemical reactions, they have distinct roles and characteristics. Catalysts often react with reactants. Difference Between Intermediates And Catalysts.
From www.finechemical.net
Catalyst vs Intermediate What is the difference? Difference Between Intermediates And Catalysts A catalyst is used at. a catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. Draw a reaction energy diagram for a given multistep process. explain the difference between a transition state and an intermediate. describe the similarities and differences between the three principal classes of catalysts. . Difference Between Intermediates And Catalysts.
From www.slideserve.com
PPT Part V Reaction Mechanisms PowerPoint Presentation Difference Between Intermediates And Catalysts the main difference between a catalyst and an intermediate is their role and status in a chemical reaction. goes over two examples that highlight the differences between catalysts and intermediates. explain the difference between a transition state and an intermediate. Catalysts often react with reactants to form intermediates that eventually yield the same reaction products and regenerate. Difference Between Intermediates And Catalysts.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Difference Between Intermediates And Catalysts the main difference between a catalyst and an intermediate is their role and status in a chemical reaction. a catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are. . Difference Between Intermediates And Catalysts.
From 2012books.lardbucket.org
Catalysis Difference Between Intermediates And Catalysts A catalyst is used at. a catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. while catalysts and intermediates both participate in chemical reactions, they have distinct roles and characteristics. goes over two examples that highlight the differences between catalysts and intermediates. explain the difference between a. Difference Between Intermediates And Catalysts.
From circuitdbnighters.z13.web.core.windows.net
Sn2 Reaction Coordinate Diagram Difference Between Intermediates And Catalysts Draw a reaction energy diagram for a given multistep process. describe the similarities and differences between the three principal classes of catalysts. Catalysts often react with reactants to form intermediates that eventually yield the same reaction products and regenerate the catalyst. while catalysts and intermediates both participate in chemical reactions, they have distinct roles and characteristics. goes. Difference Between Intermediates And Catalysts.
From quizdbcornwallis.z21.web.core.windows.net
What Is A Reaction Intermediate Difference Between Intermediates And Catalysts goes over two examples that highlight the differences between catalysts and intermediates. describe the similarities and differences between the three principal classes of catalysts. A catalyst is used at. the main difference between a catalyst and an intermediate is their role and status in a chemical reaction. while catalysts and intermediates both participate in chemical reactions,. Difference Between Intermediates And Catalysts.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Difference Between Intermediates And Catalysts the main difference between a catalyst and an intermediate is their role and status in a chemical reaction. Draw a reaction energy diagram for a given multistep process. A catalyst is used at. while catalysts and intermediates both participate in chemical reactions, they have distinct roles and characteristics. goes over two examples that highlight the differences between. Difference Between Intermediates And Catalysts.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Difference Between Intermediates And Catalysts goes over two examples that highlight the differences between catalysts and intermediates. a catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. while catalysts and intermediates both participate in chemical reactions, they have distinct roles and characteristics. A catalyst is used at. describe the similarities and differences. Difference Between Intermediates And Catalysts.
From www.slideserve.com
PPT Observing and describing chemical reactions PowerPoint Difference Between Intermediates And Catalysts Draw a reaction energy diagram for a given multistep process. goes over two examples that highlight the differences between catalysts and intermediates. Catalysts often react with reactants to form intermediates that eventually yield the same reaction products and regenerate the catalyst. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates. Difference Between Intermediates And Catalysts.
From lavelle.chem.ucla.edu
Intermediate vs. Catalyst CHEMISTRY COMMUNITY Difference Between Intermediates And Catalysts the main difference between a catalyst and an intermediate is their role and status in a chemical reaction. a catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. while catalysts and intermediates both participate in chemical reactions, they have distinct roles and characteristics. Catalysts often react with reactants. Difference Between Intermediates And Catalysts.
From www.youtube.com
Reaction Mechanisms Intermediates vs Catalysts YouTube Difference Between Intermediates And Catalysts in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are. a catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. Draw a reaction energy diagram for a given multistep process. Catalysts often react with reactants to form intermediates that eventually. Difference Between Intermediates And Catalysts.
From dxougmsaa.blob.core.windows.net
Enzymes Are Considered Catalysts And at Sylvia Pack blog Difference Between Intermediates And Catalysts the main difference between a catalyst and an intermediate is their role and status in a chemical reaction. a catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity. goes over two examples that highlight the differences between catalysts and intermediates. describe the similarities and differences between the. Difference Between Intermediates And Catalysts.
From slidetodoc.com
Section 14 6 Reaction Mechanisms and Catalysis Bill Difference Between Intermediates And Catalysts while catalysts and intermediates both participate in chemical reactions, they have distinct roles and characteristics. goes over two examples that highlight the differences between catalysts and intermediates. the main difference between a catalyst and an intermediate is their role and status in a chemical reaction. a catalyst may allow a reaction to proceed at a lower. Difference Between Intermediates And Catalysts.
From www.differencebetween.com
Difference Between Catalyst and Inhibitor Compare the Difference Difference Between Intermediates And Catalysts A catalyst is used at. goes over two examples that highlight the differences between catalysts and intermediates. in general, catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are. Draw a reaction energy diagram for a given multistep process. describe the similarities and differences between the three principal classes of. Difference Between Intermediates And Catalysts.